|
|
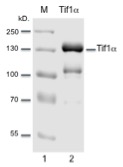
Recombinant human Tif1α (Trim24)
Catalog # : EPX-004-RBV
Source : Human
Expressed in : SF9 cells
Quantity : 10µg of
recombinant Tif1α at 0.5µg /µl
Background:
Tif1α (Trim24) is a transcriptional coactivator that
interacts with
numerous nuclear receptors and coactivators and modulates the
transcription of target genes (1, 2). Interacts with chromatin
depending on histone H3 modifications, having the highest affinity for
histone H3 that is both unmodified at 'Lys-4' (H3K4me0) and acetylated
at 'Lys-23' (H3K23ac). Has E3 protein-ubiquitin ligase activity.
Promotes ubiquitination and proteasomal degradation of p53/TP53 (3).
Plays a role in the regulation of cell proliferation and apoptosis, at
least in part via its effects on p53/TP53 levels. Up-regulates
ligand-dependent transcription activation by AR, GCR/NR3C1, thyroid
hormone receptor (TR) and ESR1. Modulates transcription activation by
retinoic acid (RA) receptors, including RARA. Plays a role in
regulating retinoic acid-dependent proliferation of hepatocytes.
Defects in TRIM24 are a cause of thyroid papillary carcinoma (4).
Protein details:
Recombinant human N-terminal FLAG tagged Tif1α was
produced in SF9
insect cells, purified using FPLC and formulated in a storage buffer
containing 20mM Tris-Cl pH 7.6, 1mM EDTA, 0.15 M NaCl, 10% glycerol,
0.5mM PMSF and 1mM DTT. Protein concentration was determined by
spectrometry. >95% purity by SDS-PAGE.
Quality control:
Each lot has been evaluated by 10% Tris Glycine SDS-PAGE.
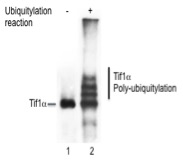
Ubiquitylation assay:
Tif1α modification with ubiquitin (Ub) was reconstituted in vitro in the presence of recombinant E1, E2, Ub and ATP. Tif1α auto-ubquitylation results in a mobility shift of 8kDa. The efficiency of in vitro auto-ubiquitylation is evaluated by 8% Tris Glycine SDS-PAGE.
Storage:
-80°C
Guarantee:
For research use only. Products guaranteed stable for 2 years from date of receipt when stored properly.
Sequence:
Tif1α (Mw: 116,831 Da.)
MEVAVEKAVAAAAAASAAASGGPSAAPSGENEAESRQGPDSERGGEAARLNLLDTCAVCH
References:
1.
Thenot et al., J. Biol. Chem. 272:12062-12068(1997) |
|---|
© 2011-2016 EpiGex. All rights reserved