|
|
Recombinant human Asf1α
Catalog # : EPX-004-REP
Source : Human
Expressed in : E. coli
Quantity : 25 µg of recombinant Asf1a at 0,5µg /µl
Protein details:
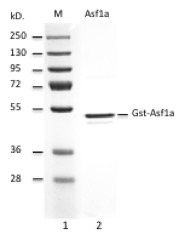
Histone chaperone that facilitates histone deposition and histone exchange and removal during nucleosome assembly and disassembly (1). Cooperates with chromatin assembly factor 1 (CAF-1) to promote replication-dependent chromatin assembly and with HIRA to promote replication-independent chromatin assembly (2, 3). Interacts with histone H3 (including both histone H3.1 and H3.3) and histone H4. Recombinant Asf1a was produced in E. coli as a N-terminal GST fusion, purified using FPLC and formulated in a storage buffer containing 20 mM Tris pH 7.65, 150 mM NaCl, 1 mM DTT, 10% glycerol. Protein concentration was determined by spectrometry. >95% purity by SDS-PAGE.
Quality control: Each lot has been evaluated by 12% Tris Glycine SDS-PAGE.
Storage:
-80°C
Guarantee:
For research use only. Products guaranteed stable for 2 years from date of receipt when stored properly.
Purity:
>98% purity by SDS PAGE.
References: 1- Munakata T., Adachi N., Yokoyama N., Kuzuhara T.,
Horikoshi M.A
human homologue of yeast anti-silencing factor has histone chaperone
activity. |
|---|
© 2011-2016 EpiGex. All rights reserved